Introduction
Although we are continuing through a difficult period with the pandemic, logistics within the pharma industry, although suffering disruption, (not to mention the effects of Brexit) continue to function well. HPRA has been active, as usual, with issuing reports and information on their observations on the Irish market.
Three recent briefs are worth reading:
- Covid Safety Update:
COVID-19 Vaccines, Overview of National Reporting Experience Report no 10
HPRA continuously monitors the vaccination programme and compiles data on reported sides effects. Some side effects have been noted, but not by every recipient, and those effects noted are generally mild in nature. Up to August 2021, a total of 13,529 reports of suspected side effects have been notified to HPRA. At that point approx. 6 million doses had been administered.
Data across the EU is collated by the European Medicines Agency who then publish safety reports which describe the various issues which are under evaluation as well as any new recommendations.
- Guide to Labels and Leaflets for Human Medicines
There has been a revision of this guide, AUT-0034-22, dated 15 July 2021.
While this revision contains the usual hygiene corrections, (typos, references etc.) there is a noteworthy change:
Labels of vaccine products and plasma-derived products. There is an addition to the requirements for peel-off labels on these products –
“The overall readability of the statutory information displayed on the fixed part of the label should not be affected by the inclusion of the peel-off part. The information provided in the peel-off label should always remain available on the fixed part of the label once the peel-off part is detached. Any exceptions to these requirements should be discussed in advance with the HPRA and will be considered on a case-by-case basis.”
The minimum information requirements for these labels remains unchanged.
- Falsified Medicines
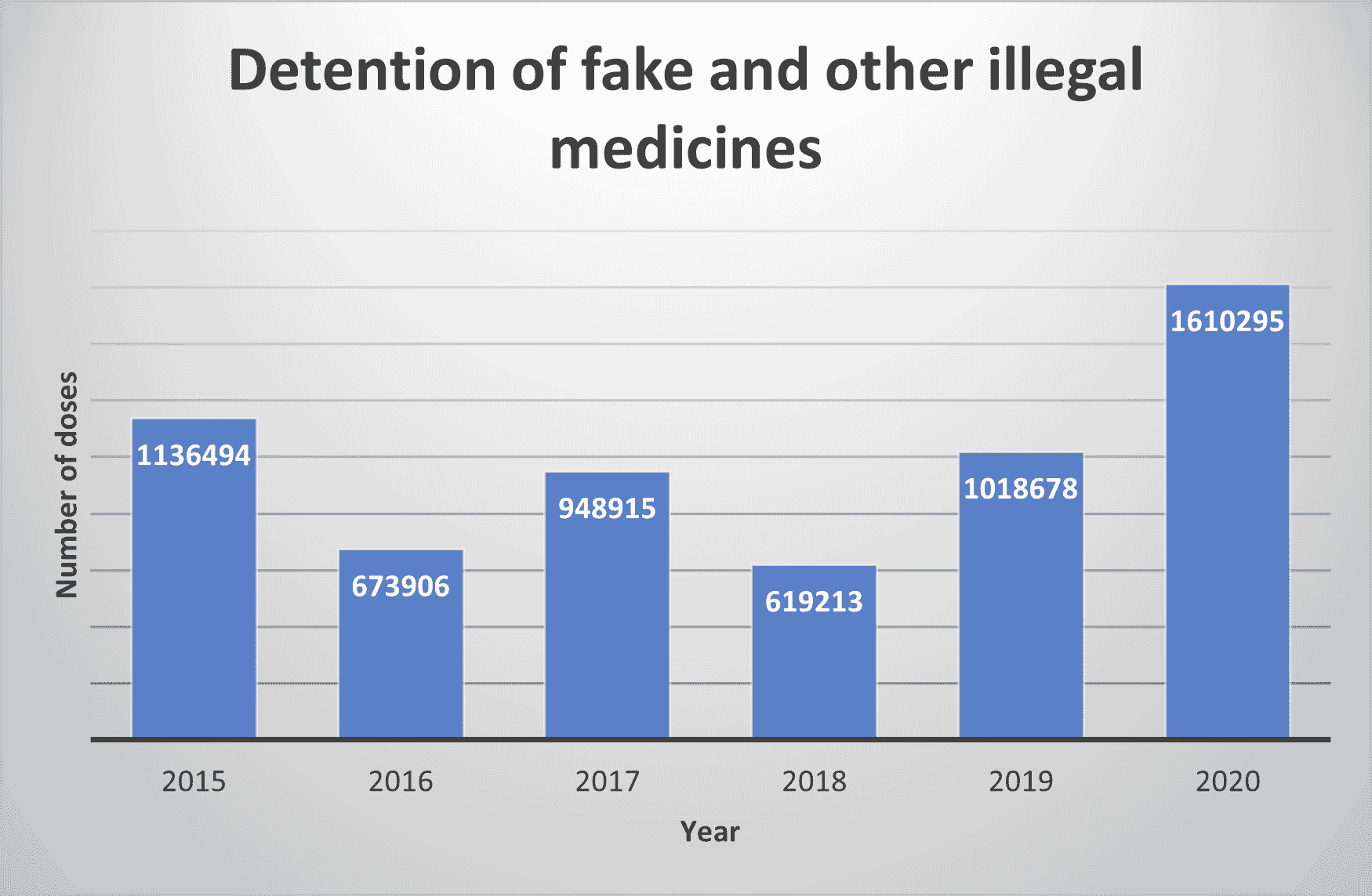
A recent (June 2020) report from the HPRA notes an increased concern with the significant rise in the number of doses of fake and illegal medicinal products detained in 2020. The increase (58% Vs 2019) shows that 1610295 does were detained, the highest number in recent years (see graph).
The report shows the breakdown by medicine type and includes, unlike previous reports, medicines other than those we’ve come to expect – Sedatives, erectile dysfunction, and Anabolic steroids. This report includes Analgesics and Covid medicines. It shows that hundreds of websites, e-commerce listings, and social media pages have been shut down. Prosecutions and formal cautions are also included.
While the internet has become the major means of procuring many products, both medicinal and non-medicinal, the procurement of prescription medicines on-line is illegal – which many people may not realise.
Nevertheless, in keeping with GDP requirements, and given all of the pandemic and Brexit impacts, vigilance is more vital than ever for those who are importing and/or distributing medicinal products for human use.
Supply Chain Enabled
